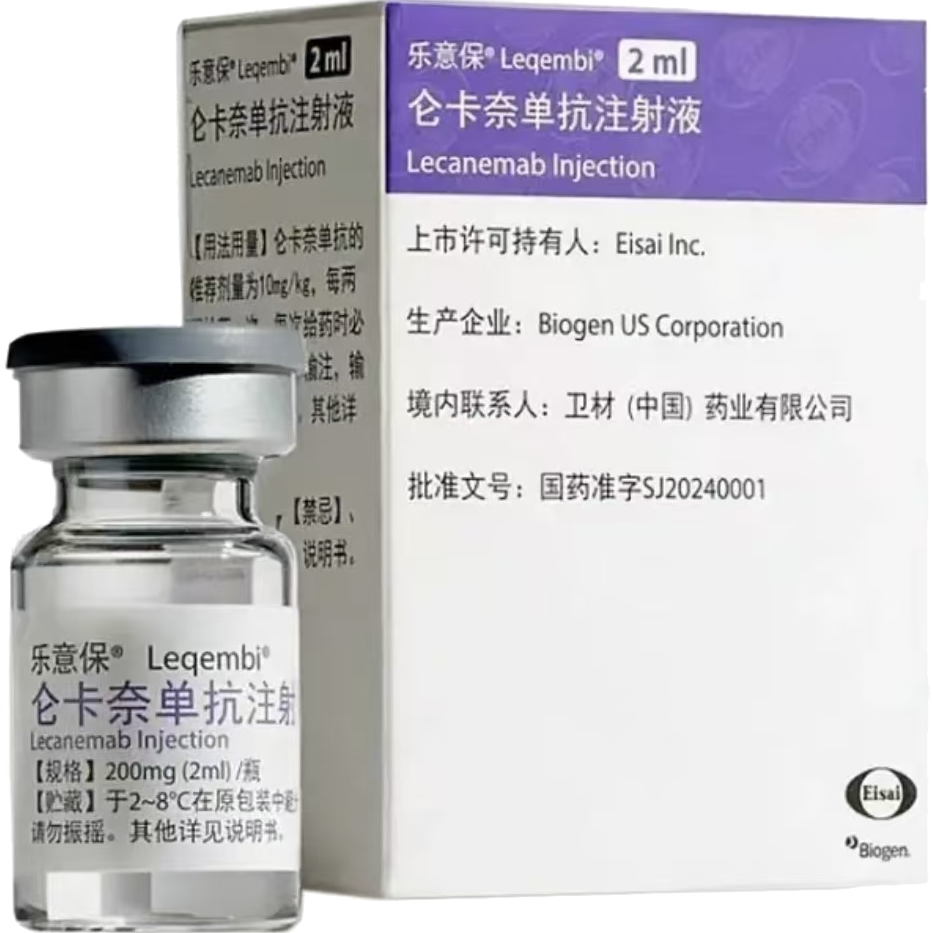
LEQEMBI
Leqembi (lecanemab) is an intravenous infusion therapy approved for the treatment of early Alzheimer's disease. It is specifically indicated for patients who meet certain criteria related to the stage of their disease and the presence of specific biomarkers.